 |
 |
- Search
| Chronobiol Med > Volume 2(1); 2020 > Article |
|
Abstract
Objective
Methods
Results
Acknowledgments
NOTES
Conflicts of Interest
Author Contributions
Conceptualization: Yu Jin Lee. Data curation: Soohyun Kim, Juhyun Park, Seong min Oh, Mi hyun Lee, Jeong Eun Jeon, Ha Young Lee. Formal analysis: Soohyun Kim. Funding acquisition: Yu Jin Lee. Investigation: Soohyun Kim, Juhyun Park, Seong min Oh, Mi hyun Lee, Jeong Eun Jeon, Ha Young Lee. Project administration: Yu Jin Lee. Resources: Yu Jin Lee. Supervision: Yu Jin Lee. Visualization: Soohyun Kim. Writing—original draft: Soohyun Kim. Writing—review & editing: Soohyun Kim, Yu Jin Lee.
Figure 1.

Figure 2.
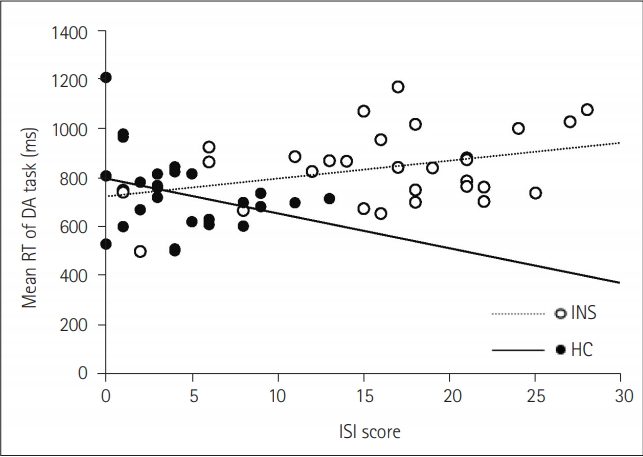
Figure 3.
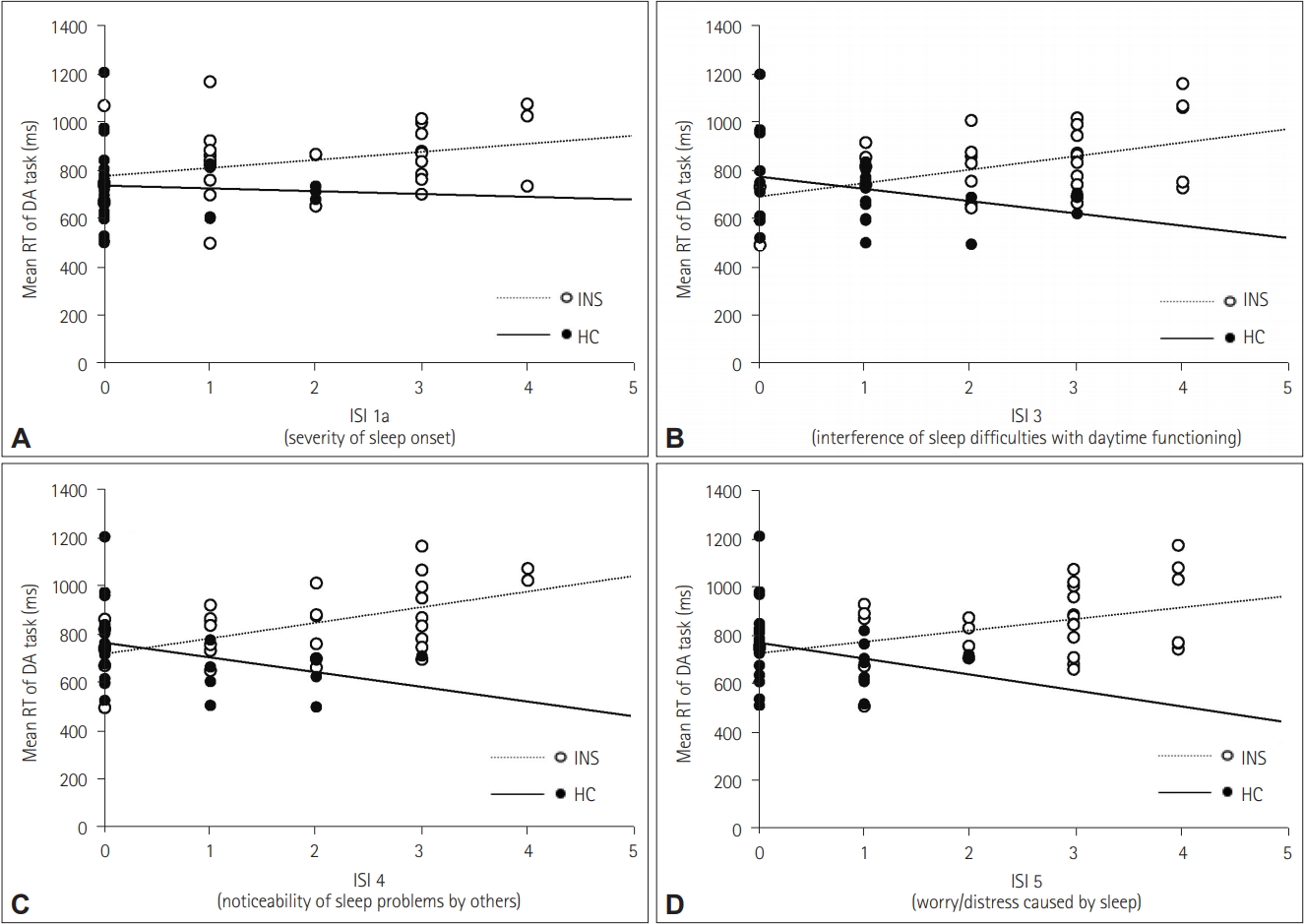
Figure 4.
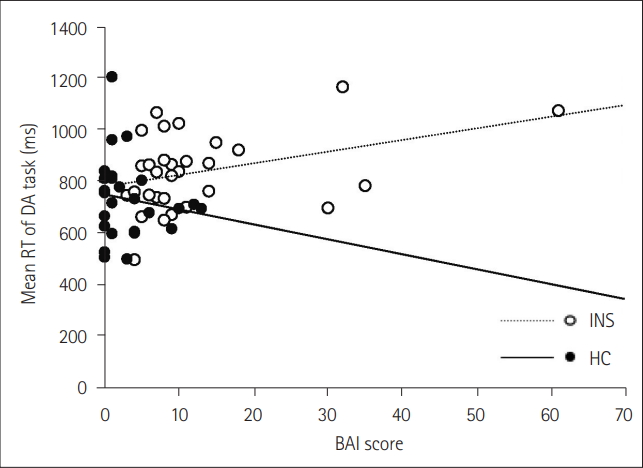
Table 1.
| INS (n=30) | HC (n=26) | F | |
|---|---|---|---|
| Demographic | |||
| Age (yr) | 43.87±13.73 | 37.08±13.40 | |
| Gender (F/M) | 24/6 | 13/13 | |
| Attention performance | |||
| SA | |||
| Number of OE | 0.60±1.10 | 1.31±3.52 | 2.50 |
| Number of CE | 6.70±5.87 | 6.85±7.50 | 0.20 |
| Mean RT (ms) | 457.35±77.10 | 412.49±62.73 | 3.62† |
| DA | |||
| Number of OE | 9.00±7.35 | 10.77±10.12 | 0.97 |
| Number of CE | 4.60±6.83 | 3.73±3.85 | 0.48 |
| Mean RT (ms) | 838.25±147.91 | 733.21±156.16 | 3.80† |
| Questionnaire | |||
| ESS | 6.77±4.26 | 6.85±3.82 | 0.20 |
| PSQI | 10.87±3.73 | 4.85±2.07 | 38.72* |
| ISI | 15.83±7.36 | 4.42±3.49 | 37.58* |
| DBAS-16 | 158.03±37.52 | 125.31±29.72 | 7.65* |
| BDI | 12.07±10.60 | 3.00±4.02 | 13.17* |
| BAI | 12.63±12.18 | 3.12±3.89 | 9.34* |
| Polysomnography | |||
| TST (min) | 400.88±55.98 | 431.24±38.45 | 2.67 |
| SE (%) | 85.95±11.38 | 90.68±7.01 | 0.93 |
| WASO (min) | 52.10±51.13 | 33.90±33.62 | 0.37 |
| % stage N1 | 12.00±4.65 | 11.85±5.54 | 0.36 |
| % stage N2 | 56.49±9.03 | 58.01±9.12 | 0.71 |
| % stage N3 | 10.06±7.73 | 8.70±6.00 | 0.91 |
| % stage REM | 26.72±27.43 | 21.45±5.88 | 0.74 |
| SL (min) | 12.40±19.35 | 10.18±7.27 | 0.16 |
| REML (min) | 91.76±46.36 | 111.26±48.07 | 2.37 |
| AHI (/hr) | 2.86±2.77 | 3.56±4.03 | 0.24 |
| PLMI (/hr) | 2.36±3.78 | 2.10±3.63 | 0.03 |
INS: patients with insomnia disorder, HC: healthy control, OE: omission error, CE: commission error, RT: reaction time, SA: sustained attention, DA: divided attention, ESS: Epworth Sleepiness Scales, PSQI: Pittsburgh Sleep Quality Index, ISI: Insomnia Severity Index, DBAS-16: Dysfunctional Beliefs and Attitudes about Sleep Scale 16, BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory, TST: total sleep time, SE: sleep efficiency, WASO: wake after sleep onset, SL: sleep latency, REML: REM latency, AHI: Apnea–Hypopnea Index, PLMI: Periodic Limb Movement Index
Table 2.
|
Sustained attention (r) |
Divided attention (r) |
|||
|---|---|---|---|---|
| INS (n=30) | HC (n=26) | INS (n=30) | HC (n=26) | |
| Questionnaire | ||||
| ESS | 0.11 | -0.02 | -0.01 | 0.00 |
| PSQI | 0.17 | -0.37 | 0.18 | -0.38 |
| PSQI component 1 | 0.15 | -0.28 | 0.17 | -0.46* |
| PSQI component 2 | 0.04 | -0.24 | 0.00 | -0.09 |
| PSQI component 3 | 0.03 | -0.06 | 0.14 | -0.22 |
| PSQI component 4 | 0.02 | -0.32 | 0.01 | -0.21 |
| PSQI component 5 | -0.10 | -0.17 | 0.11 | -0.34 |
| PSQI component 6 | 0.33 | -0.16 | 0.30 | -0.09 |
| PSQI component 7 | 0.12 | -0.33 | -0.05 | -0.23 |
| ISI | 0.22 | -0.30 | 0.40* | -0.58* |
| ISI 1a | 0.14 | -0.21 | 0.38* | -0.30 |
| ISI 1b | 0.04 | -0.31 | 0.06 | -0.31 |
| ISI 1c | 0.06 | -0.40 | 0.13 | -0.29 |
| ISI 2 | 0.30 | -0.19 | 0.35 | -0.21 |
| ISI 3 | 0.16 | -0.01 | 0.44* | -0.42* |
| ISI 4 | 0.35 | 0.01 | 0.57* | -0.35 |
| ISI 5 | 0.22 | -0.25 | 0.44* | -0.47* |
| DBAS-16 | -0.24 | 0.09 | 0.16 | 0.12 |
| BDI | 0.01 | -0.18 | 0.29 | -0.32 |
| BAI | -0.09 | -0.25 | 0.39* | -0.28 |
| Polysomnography | ||||
| TST (min) | 0.04 | 0.38 | -0.17 | 0.30 |
| SE (%) | -0.03 | 0.42* | -0.27 | 0.25 |
| WASO (min) | 0.11 | -0.40 | 0.18 | -0.20 |
| % stage N1 | 0.13 | 0.11 | 0.22 | -0.26 |
| % stage N2 | 0.10 | 0.00 | 0.10 | -0.35 |
| % stage N3 | -0.14 | -0.17 | -0.06 | 0.58* |
| % stage REM | -0.03 | 0.12 | 0.39* | 0.11 |
| SL (min) | 0.04 | -0.19 | 0.44* | -0.20 |
| REML (min) | 0.10 | -0.17 | 0.20 | -0.09 |
| AHI (/hr) | -0.15 | -0.06 | 0.01 | -0.11 |
| PLMI (/hr) | -0.19 | -0.09 | -0.33 | -0.15 |
INS: patients with insomnia disorder, HC: healthy control, ESS: Epworth Sleepiness Scales, PSQI: Pittsburgh Sleep Quality Index, ISI: Insomnia Severity Index, DBAS-16: Dysfunctional Beliefs and Attitudes about Sleep Scale 16, BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory, TST: total sleep time, SE: sleep efficiency, WASO: wake after sleep onset, SL: sleep latency, REML: REM latency, Apnea–Hypopnea Index, PLMI: Periodic Limb Movement Index
REFERENCES
-
METRICS

- Related articles in Chronobiol Med
-
Sleep-Related Attentional Bias to Word Stimuli in Patients with Insomnia Disorder2019 June;1(2)






