Relationship Between Anxiety and Heart Rate Variability in Elderly Insomnia Patients
Article information
Abstract
Objective
This study investigated the association between anxiety symptoms in elderly insomnia patients and heart rate variability (HRV) parameters.
Methods
In total, 46 elderly patients (67.91±5.61 years old, 11 males, 35 females) were enrolled. The Pittsburgh Sleep Quality Index, Beck Anxiety Inventory, Albany Panic and Phobia Questionnaire (APPQ), Penn State Worry Questionnaire, and Liebowitz Social Anxiety Scale were used to measure sleep quality and anxiety. Resting-state HRV was also obtained. The associations between anxiety measures and HRV were analyzed.
Results
Female sex was related to higher normalized high-frequency (HF norm) power (β=0.32, p=0.04). The APPQ score was negatively correlated with the HF norm (β=-0.59, p<0.01). Age was negatively correlated with low-frequency power/high-frequency power (LF/HF) ratio (β=-0.33, p=0.04). Female sex was correlated to lower LF/HF ratio (β=-0.36, p=0.02). The APPQ score was positively correlated with LF/HF ratio (β=0.68, p<0.01).
Conclusion
Panic symptoms in elderly insomnia patients were negatively correlated with parasympathetic nervous system activity.
INTRODUCTION
Sleep problems are common in the elderly [1] and insomnia is the most frequently reported sleep problem [2]. Insomnia is broadly defined as subjective sleep dissatisfaction [1]. The prevalence of insomnia in the elderly is estimated to be 40%–50%, exceeding that of their younger counterparts [3]. As the population is getting older [4], the burden of insomnia could become more extensive.
Efforts have been made to investigate the role of the autonomic nervous system in insomnia [5]. Some researchers have measured daytime resting-state heart rate variability (HRV) in insomnia patients. Fang et al. [6] compared frequency domain HRV between insomnia patients and healthy individuals and reported a tendency toward lower high-frequency (HF) power in insomniacs. Cellini et al. [7] measured autonomic nervous system activity in insomnia patients and reported a decrease in HF power, suggesting decreased parasympathetic nervous system activity despite no significant between-group difference in low-frequency (LF) power or the LF power/HF power (LF/HF) ratio.
An association between anxiety disorder and HRV has been repeatedly reported [8]. In a meta-analysis, encompassing 36 studies, a comparison of HRV between anxiety patients and healthy controls resulted in a lower HF power and lower time domain parameters in the anxiety samples. In further analyses, HRV was analyzed in a specific subset of anxiety disorders, i.e., panic disorder, post-traumatic stress disorder, generalized anxiety disorder, social anxiety disorder, and obsessive-compulsive disorder. All anxiety disorders, excluding obsessive-compulsive disorder, demonstrated a decrease in HF power, suggesting impaired parasympathetic activity [8].
Previous studies have reported a close relationship between insomnia and anxiety. A prospective study revealed that anxiety and insomnia are bidirectionally and temporally related, though that study excluded older adults [9]. Another study investigated self-reported anxiety symptoms in insomniacs and detected an association between insomnia and anxiety [10]. Unfortunately, no study has investigated autonomic nervous system activity in elderly insomniacs with comorbid anxiety. This issue has primary importance, as it could be a key to discriminating specific phenotypes from a heterogeneous insomnia group, facilitating future studies on the etiology and treatment of insomnia [11].
We investigated the association between anxiety symptoms in elderly insomnia patients and HRV parameters as an indicator of autonomic nervous system activity. We hypothesized that an elevated anxiety level in elderly insomnia patients would be associated with a decrease in HF power and an increase in the LF/HF ratio.
METHODS
Participants
The participants were recruited from Samsung Medical Center, between November 10, 2021, and October 11, 2022. The study participants were recruited among visitors to the Department of Psychiatry, Samsung Medical Center. The inclusion criteria were age ≥60 years and suffering from insomnia. The exclusion criterion was an inability to complete the questionnaires (e.g., intellectual disability, major neurocognitive disorder, severely impaired schizophrenia, bipolar disorder, history of substance abuse in recent years, physical illness affecting sleep, epilepsy affecting electroencephalography, cardiac disease affecting electrocardiography results, or shift work).
Initially, 50 participants (68.04±5.70 years, 12 males, and 38 females) were recruited. After the initial recruitment, four participants dropped out, as they did not complete the questionnaires. Finally, 46 elderly patients (67.91±5.61 years old, 11 males, and 35 females) were included in the study. The mean number of educational years of the study participants was 12.46±3.59. Overall, 15 (32.6%) participants were currently employed and 28 (60.8%) were unemployed. Further, 38 (82.6%) subjects were married, 4 (8.7%) were divorced, and 3 (6.5%) were single (Table 1).
All study procedures were carried out following the 1964 Declaration of Helsinki as revised in 2013. This study protocol was approved by the Institutional Review Board of Samsung Medical Center (protocol code 2021-11-043). All participants provided informed consent.
Questionnaires
Demographic data and the sleep and anxiety questionnaires were obtained by self-report survey. The Korean version of the Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality [12]. The PSQI contains seven domains (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction), summed up in a global index of sleep quality [12]. The Korean version of the PSQI has high internal consistency, reliability, and validity [13].
The Korean version of the Beck Anxiety Inventory (BAI) was used to measure overall anxiety symptoms in the study subjects [14]. The BAI contains 21 items, each measuring anxiety and distress [14]. A higher BAI score indicates more severe anxiety [14]. A previous study verified the internal consistency and test-retest reliability of the Korean version of the BAI [15].
The Korean version of the Albany Panic and Phobia Questionnaire (APPQ) was used to measure panic and phobic anxiety [16]. This 27-item questionnaire is designed to measure agoraphobia, social phobia, and interoceptive fears [16]. The Korean version of the APPQ demonstrated good internal consistency and test-retest reliability [17].
The Penn State Worry Questionnaire (PSWQ) contains 16 items that capture the specific trait of worry [18]. A higher score on the PSWQ means a higher level of trait worry [18]. The Korean version of the PSWQ demonstrates good internal consistency and test-retest reliability [19].
The Liebowitz Social Anxiety Scale (LSAS) was designed to measure social anxiety levels [20]. The LSAS contains 24 socially engaging situations and evaluates the levels of fear and avoidance in each situation [20]. The Korean version of the LSAS demonstrates good internal consistency [21].
HRV measurement
Resting-state HRV was obtained. HRV captures the beat-to-beat variance in the heart rate. As the heart rate is modulated by the autonomic nervous system, HRV theoretically reflects autonomic function. Several domains of HRV measurement, including the time domain, frequency domain, non-linear measures, and heart rate turbulence were considered. In this study, we considered frequency domain. The frequency domain is a parameter that measures heart rate periodicity during a particular time interval. LF power is the variation in heart rate every 6.7 s to every 25 s (0.04–0.15 Hz), reflecting the functions of the sympathetic and parasympathetic nervous systems. HF power is the heart rate variation every 2.5 s to 6.7 s (0.15–0.40 Hz). HF power reflects the activity of the parasympathetic nervous system. Thus, the LF/HF ratio is assumed to indicate relative sympathetic activity compared to parasympathetic activity. The normalized HF power (HF norm) is the HF power divided by the total power (TP) minus the very low frequency power and reflects parasympathetic nervous system activity [22].
Statistical analyses
The demographic characteristics of the study participants were described as means and standard deviations. A multiple linear regression analysis was used to detect the associations between anxiety symptoms (BAI, APPQ, PSWQ, and LSAS) and HRV parameters (TP, HF norm, and LF/HF ratio). Other variables (age, sex, and the PSQI) were included in the model as independent variables considering their potential relationship with the HRV parameters. The analysis was conducted using SPSS version 27.0 software (IBM Corp., Armonk, NY, USA). A p-value ≤0.05 was considered significant.
RESULTS
Psychiatric characteristics and HRV parameters
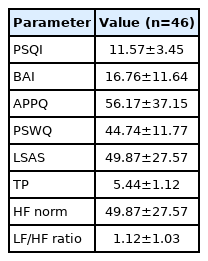
The mean PSQI score was 11.57±3.45. The mean BAI score was 16.76±11.64. The mean APPQ score was 56.17±37.15. The mean PSWQ score was 44.74±11.77, and the mean LSAS score was 49.87±27.57. The HRV measurements were a TP of 5.44±1.12, HF norm of 49.87±27.57, and an LF/HF ratio of 1.12±1.03 (Table 2).
Correlations between anxiety and the HRV parameters
None of the independent variables demonstrated associations with TP. In further analyses, sex and the APPQ score were correlated with HF norm. Female sex was correlated with a higher HF norm (β=0.32, p=0.04). The APPQ score was negatively correlated with HF norm (β=-0.59, p<0.01) (Figure 1). Age, sex, and APPQ score were correlated with the LF/HF ratio. Age was negatively correlated with the LF/HF ratio (β=-0.33, p=0.04). Female sex was correlated with a lower LF/HF ratio (β=-0.36, p=0.02). The APPQ score was positively correlated with the LF/HF ratio (β=0.68, p<0.01). In contrast, the PSQI score and other anxiety measures (BAI, PSWQ, and LSAS) were not associated with any of the HRV parameters (Table 3).
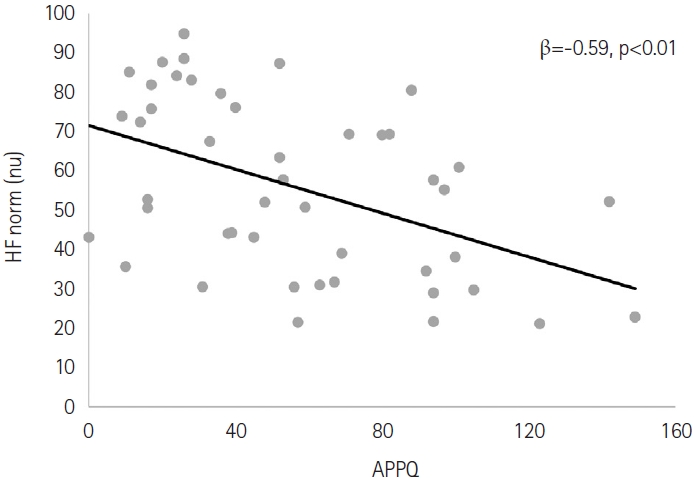
Correlation between the Albany Panic and Phobia Questionnaire (APPQ) score and normalized high-frequency (HF norm) power. nu, normalized unit.
DISCUSSION
The current study reports HRV measurements and anxiety symptoms in elderly insomnia patients. Among the anxiety symptoms, panic and phobia symptoms were correlated with a low HF norm, suggesting decreased parasympathetic activity. In contrast, the worry trait and social anxiety did not accompany the change in HRV. In addition, decreased sleep quality did not accompany certain changes in HRV.
Our result, suggesting an association between panic symptoms and low HRV, is consistent with previous studies. Most studies have compared HRV measurement between patients with panic disorder and healthy controls [23-25]. The present study suggests a possible dose-dependent relationship between the panic index and HRV parameters in elderly people with insomnia. This result suggests that the presence of a specific disease entity, such as insomnia comorbid with anxiety, accompanies decreased parasympathetic output activity.
In contrast to panic symptoms, the overall anxiety index, the worry trait, and social anxiety were not associated with changes in HRV. This result differs from previous research [24,26]. It is possible that aging masked the changes in HRV associated with anxiety symptoms. In a previous study, aging was associated with a specific pattern of HRV change, i.e., a U-shaped pattern with the nadir at the seventh decade [26]. Considering the mean age (67.91±5.61 years old) of the participants, a decrease in HF power related to age could decrease the change in HF power accompanied by anxiety symptoms. Similarly, lower sleep quality was not associated with the change in HRV in the present study, as opposed to a previous report [27]. This outcome could also be explained by an effect of aging on HRV measurements.
In addition, aging was associated with a decrease in sympathovagal balance, which was partially explained by a previous study suggesting an increase in parasympathetic output after 60 years of age [26]. Furthermore, female participants were associated with higher parasympathetic output than males, consistent with a previous study [26].
The current study had some limitations. This study was limited by single-center enrollment and a small sample size. However, this study is significant as it included a specific population of elderly insomnia patients. In addition, the current study did not include healthy controls. However, as many case-control studies have been conducted, the current study intended to investigate the dose-dependent effect of anxiety on HRV parameters to expand the findings of previous research. The present study was also limited by the HRV measurements. We only measured resting-state HRV, although it is likely that HRV measurements during sleep might display another pattern in elderly insomniacs comorbid with anxiety. Additional studies investigating the association between sleep state HRV and anxiety in elderly insomniacs may be needed. In addition, the medications of the participants were not considered in the current study, although medication could affect HRV parameters.
In conclusion, panic symptoms in elderly insomnia patients were negatively correlated with parasympathetic nervous system activity. In contrast, the worry trait, social anxiety, and sleep quality were not associated with changes in the HRV parameters in elderly insomnia patients.
Notes
Funding Statement
This research was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (RS-2020-KD000188) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HR21C0885).
The authors have no potential conflicts of interest to disclose.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Jin Won Seo. Data curation: Minjeong Kim. Formal analysis: Jin Won Seo. Funding acquisition: Seog Ju Kim. Investigation: Minjeong Kim, Hyerin Gu, Hyeyeon Jang, Jin Won Seo. Methodology: Jin Won Seo. Project administration: Seog Ju Kim. Resources: Seog Ju Kim. Software: Minjeong Kim, Jin Won Seo. Supervision: Seog Ju Kim. Validation: Jin Won Seo, Seog Ju Kim. Visualization: Jin Won Seo. Writing—original draft: Jin Won Seo. Writing—review & editing: Seog Ju Kim.