A Meta-Analysis of the Effectiveness of Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation as Therapy of Insomnia
Article information
Abstract
Objective
Insomnia serves as the most common sleep disorder. Transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) are amongst most popular treatment for insomnia. This research aimed to determine the comparative effectiveness of TMS and tDCS as therapies for insomnia.
Methods
PubMed/MEDLINE, ScienceDirect, Google Scholar, ResearchGate, Wiley Library Online, and Neurona were used to search for randomized controlled trials (RCTs) comparing TMS/tDCS with sham group in insomnia. Meta-analysis was done to determine the effectiveness of TMS and tDCS as a therapy for insomnia. Weighted mean difference were computed and reported with its 95% CI. Heterogeneity was reported with I2. Risk of bias was assessed using the Cochrane Risk of Bias 2 tool. Subjective pooled-analysis was done using Mann-Whitney U test with p<0.05.
Results
From a total 1,000 publications, 15 studies were included, revealing varying types of insomnia as follows: 26.7% comorbid depression, 13.3% comorbid pain, 13.3% involving athletes, 13.3% primary insomnia, 6.7% due to medication, 6.7% comorbid polio, 6.7% comorbid Sjogren’s syndrome, 6.7% comorbid stroke, and 6.7% comorbid Parkinson’s disease. From 15 studies, four homogenous articles were made for pooled-analyses, which included two studies on TMS and two studies on tDCS in insomnia comorbid depression. tDCS improved sleep onset latency, N2, and REM latency better than sham. TMS (2.29, 95% CI: 1.82–2.76) was superior to sham in improving the subjective quality of sleep, and tDCS (1.05, 0.68–1.43) with p=0.121.
Conclusion
No significant differences were found between TMS and tDCS as therapies for insomnia. Most RCTs studying TMS/tDCS in insomnia reported high comorbidity with depression.
INTRODUCTION
Almost all people in the world may have had trouble of sleeping, and insomnia serve as the most prevalent sleep disorder. Based on the International Classification of Sleep Disorders– Third Edition, insomnia is defined as subjective perception of difficulty initiating sleep, maintaining sleep, shortening sleep duration, and decreasing sleep quality, even though patients have adequate time to sleep. Insomnia usually reported as obtained sleep latency longer than 30 minutes, waking after sleep onset longer than 30 minutes, sleep efficiency less than 85%, or total length of sleep less than 6.5 hours, occurring at least 3 days a week [1-4]. International Classification of Disease, 10th Revision (ICD-10) and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) both further categorize insomnia into primary and secondary. Secondary insomnia occurs alongside underlying comorbidities often present as changes in circadian rhythm, daily behaviors, environmental factors, other sleep disorders, movement disorders, medical conditions, neurological disorders, psychiatric disorders, menstruation, pregnancy, medication use, drug dependence, and various other complaints [1,3].
Patient with insomnia shows persistent hyperarousal and disruptions in connectivity within the default mode network (DMN) in the medial frontal gyrus and the salience network (SN) in the insula, which are related to subjective sleep disturbances, hyperarousal, maladaptive emotional regulation, and emotional state integration disorders. The DMN is active during rest and inactive during activity. In addition, functional connectivity disruptions are also found in the amygdala, posterior cingulate gyrus, insula, and hippocampus in insomnia. Hyperarousal is reported in insomnia patient both during sleep and awake. Difficulty in falling asleep and staying asleep are associated with functional hyperconnectivity [5,6].
The primary goal of treatment is to improve sleep quality and quantity while reducing daytime complaints associated with insomnia. Medication therapy is an option to assist with initial management if needed. Short-acting medications are typically chosen for initial management, while long-acting medications are only prescribed if initial management is unsuccessful. Sometimes a combination of therapies is required. Other non-pharmacological therapies include relaxation techniques with music, bright light exposure in the morning, acupuncture or acupressure, meditationbased movement interventions, and brain stimulation [2,3,7,8].
Non-invasive brain stimulation (NIBS) is defined as brain activity modulation using specific techniques that do not require invasive maneuvers or instrumentation on the body [9]. Transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) are the two most popular types of NIBS and can be used for insomnia [10-13]. TMS is a tool capable of delivering extracranial electromagnetic waves that can pass through the skull or cranium. In adequate amounts, these waves can depolarize or excite neurons in the cortex [2]. TMS can induce sleep waves, thereby improving sleep quality, optimizing sleep structure, and maintaining therapeutic efficacy to a greater extent than pharmacological treatments and cognitive-behavioral interventions [6,14]. Transcranial DCS uses low-intensity electrical current (1–2 mA) delivered through two electrodes with the application of positive (anodal) or negative (cathodal) currents placed on the scalp to modulate neuron activity and induce modulation of the membrane potentials of neurons in the underlying cortex [15-17]. The frontal cortex is the target area for NIBS in insomnia, especially in the dorsolateral prefrontal cortex (DLPFC) which identified in the international 10–20 system [6,12].
The potential mechanism of TMS in improving sleep quality in patients with insomnia involves inhibiting hyperarousal states in the cerebral cortex. Both the right and left prefrontal cortex play crucial roles in regulating sleep in individuals with poor sleep quality and insomnia. One reason is that NIBS stimulation at the DLPFC location induces top-down sleep-wake regulation pathways [18-21]. Impairment in insular activity and abnormal connectivity also occur in insomnia, and insula is a critical brain region within the salience network. Reduced functional connectivity within the thalamus, a prominent area in hyperarousal system, and decreased functional connectivity in the medial frontal gyrus, a core region of the DMN, are observed in insomnia. TMS can provide therapeutic effects by reducing spontaneous hyperconnectivity in the DMN, insula, and thalamus, thereby allowing thalamus to block sensory signal transmission from the ARAS and thalamus to the cerebral cortex [6]. In insomnia comorbid depression, both excitatory (glutamate) and inhibitory (GABA) pathways are involved and TMS is known can significantly decrease glutamate [22]. Additionally, there is evidence to suggest that DLPFC stimulation by TMS can induce the release of dopamine and melatonin, increase serotonin and noradrenaline concentrations, as well as serum levels of GABA and BDNF which are major neurotransmitters in the sleep-wake cycle [13].
The mechanism of action of tDCS on DLPFC region involves modulating cortical stimulation, affect the top-down pathway that targets the corticothalamic pathway in regulating sleep-wake cycles. This modulation helps reduce hyperarousal and hyperactivity in the DMN and SN regions, leading to a decrease in sleep onset latency (SOL) and an increase in total sleep time (TST) [18,23]. It also triggers sleep waves and sleep spindles, especially in N2, and reduces the likelihood of transitioning from N2 sleep to wakefulness while increasing the transition from N2 to N3 [24,25].
Previous research conducted on the effects of TMS and tDCS interventions on insomnia has provided valuable insights into each intervention method. However, to the best of our knowledge, there has been no research comparing the effectiveness of TMS and tDCS as therapies for insomnia. Therefore, there is a clear need to fill the knowledge gap related to the direct comparison between TMS and tDCS as therapy for insomnia. Furthermore, the scarcity of randomized controlled trial (RCT) studies that have focused on insomnia is a deficiency that needs to be addressed. By conducting this research, we can fill this knowledge gap and make a significant contribution by conducting the most extensive RCT study on the type of insomnia that considers the effectiveness of TMS and tDCS for treating insomnia. Through this initiative, we will be able to provide a stronger empirical foundation to guide clinical practices in the treatment of insomnia. Our aim is to review the effectiveness of TMS and tDCS as treatments for insomnia and investigate the differences between them.
METHODS
Literature search for RCT was done through PubMed/MEDLINE, ScienceDirect, ResearchGate, Wiley Online Library, Google Scholar, and Neurona. The search for articles was limited to Bahasa and English with no restrictions for publication years, and design is based on recommendations from PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). This study has been registered with PROSPERO (www.crd.york.ac.uk/prospero/) under the reference number CRD42023457693.
We included studies with following criterias: 1) Participants: all individuals with insomnia based on clinical diagnosis with subjective or objective complaints of insomnia; 2) Intervention: TMS/tDCS; 3) Comparison: sham group; and 4) Outcome: the researchers included research outcomes related to improvements in insomnia assessed based on polysomnography (PSG) parameters such as SOL, TST, wake after sleep onset (WASO), sleep efficiency (SE), or subjective sleep quality which assessed by using the Pittsburgh Sleep Quality Index (PSQI) or other insomnia-related parameters.
We extracted data based on the year of publication, year of data collection, study design, country of origin, type of insomnia, diagnosis, treatment parameters, number of intervention sessions, stimulation location, outcomes, and sleep quality. To extract the data, DM and WW used the “Covidence” software (www.covidence.org). We conducted the initial screening by examining titles, abstracts, sample sizes, and outcome parameters then conducted a more thorough review of the title, abstract, and full-text of research articles.
Risk of bias was assessed using Traffic Light Plots RoB 2 [26] for each study, utilizing a table format. Potential bias risks that may arise in the analysis include bias in the randomization process, deviations from the intended intervention, missing data on study outcomes, outcome measurement, and outcome reporting selection.
Data was analyzed using SPSS 28 (IBM Corp., Armonk, NY, USA). For continuous data, mean difference (MD) was formulated if measured on the same scale, and standardized mean difference (SMD) was formulated if measured on different scales. Dichotomous data results were reported as risk ratios, odds ratios, or risk differences. A 95% confidence interval (CI) and p<0.05 was used. To assess heterogeneity in the studies, chi-squared test and I2 statistic were employed (0%–40%: low heterogeneity; 30%– 60%: moderate heterogeneity; 50%–90%: substantial heterogeneity; 75%–100%: high heterogeneity). In an effort to assess the stability of the meta-analysis results in the face of changes, sensitivity analyses were conducted. This may involve identifying publication bias and conducting tests for special conditions based on studies that do not fully meet the inclusion criteria and significantly influence the overall results. If a study does not meet the inclusion criteria or has a substantial impact on the overall results, it was used in the analysis.
Detailed description of sample characteristics in each research article as well as the study outcomes were presented. For all outcomes, the researchers summarized the results of TMS and tDCS therapy for individuals with insomnia, including confidence intervals and bias risk. Forest plots were used to visualize the estimates from each study and the combined estimates resulting from the systematic review. Each research article was evaluated for its quality using the Grading Recommendation Assessment, Development, and Evaluation (GRADE) criteria, with the application of GRADEpro (www.gradepro.org). Publication bias evaluation is conducted using a funnel plot.
RESULTS
Literature search yielded 1,000 articles, and 135 duplicate articles were excluded. After screening based on titles and abstracts, 865 articles remained. Of these, 850 articles did not meet the inclusion criteria, leaving 15 full-text articles for eligibility assessment. These articles covered various types of insomnia, with the distribution as follows: 26.7% comorbid depression insomnia, 13.3% with comorbid pain, 13.3% insomnia in athletes, 13.3% primary insomnia, 6.7% medication-induced insomnia, 6.7% comorbid polio, 6.7% comorbid Sjogren’s syndrome, 6.7% comorbid stroke, and 6.7% comorbid Parkinson’s disease. Because of various types of insomnia, it was a challenge of determining the types of insomnia suitable for conducting a meta-analysis due to the high level of heterogeneity among the article samples which could make significant bias. Therefore, for this study, meta-analysis was conducted on the most homogeneous articles reporting similar comparison/control and outcome with the most common type of insomnia which is insomnia comorbid with depression at 26.7%, which contained four research articles [27-30] with two studies using TMS and two studies using tDCS, all of the studies are from China (Figure 1). A summary of the characteristics of TMS and tDCS is provided in Table 1 and quality assessment in Figure 2.

Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the systematic review and meta- analysis.

Grading Recommendation Assessment, Development, and Evaluation (GRADE) assessment for quality of evidence. TMS, transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; PSQI, Pittsburgh Sleep Quality Index; CI, confidence interval; HAMD, Hamilton Depression Rating Scale; SOL, sleep onset latency; TST, total sleep time; NREM, non-rapid eye movement; REM, rapid eye movement.
Bias assessment was conducted using Traffic Light Plots RoB 2, and overall risk of bias from gathered research articles were low (Figure 3). The quality of the research was assessed using the GradePro software and the overall analysis results were provided for all selected articles. The quality of the outcomes was evaluated for each selected article by assessing components such as bias risk, inconsistency, indirectness, and precision. Based on the risk of bias assessment, all the studies had a low risk of bias because they adhered to the inclusion criteria in their study designs. Outcome assessment was evaluated in each selected article by assessing components such as bias risk, inconsistency, indirectness, and imprecision, leading to high certainty in the conclusions drawn from each outcome. This thorough assessment of bias and research quality ensures that the findings of the selected articles are reliable and credible.

Assessment for risk of bias. TMS, transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
The meta-analysis of the subjective sleep quality assessment using the PSQI was conducted in TMS studies during the fourth week. The results showed a weighted mean difference (WMD) of 2.29 (95% CI: 1.82 to 2.76), with p<0.001 and I2=91% (Figure 4). The subjective assessment of sleep quality in tDCS studies using components of the PSQI and sleep-related components of the Hamilton Depression Rating Scale (HAMD) was conducted after the intervention. The results showed a WMD of 1.05 (95% CI: 0.68 to 1.43), p<0.001 and I2=91% (Figure 4).

Forest plot subjective sleep. TMS, transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
The meta-analysis of the objective sleep parameter assessment using PSG was used in both active tDCS and sham group to assess objective sleep parameters in individuals with insomnia. The following results were obtained for various sleep parameters: 1) SOL: WMD 4.39 (95% CI: 3.61 to 5.16), p<0.001, and an I2=99%. This indicates a reduction in SOL in the group receiving tDCS therapy compared to the sham group; 2) TST: WMD 1.80 (95% CI: 1.35 to 2.24), p<0.001, and an I2=98%. This suggests an increase in TST in the tDCS therapy group compared to the sham group; 3) NREM sleep phases: N1: WMD -2.58 (95% CI: -3.05 to -2.10), p<0.001, and an I2=89%. This indicates a reduction in N1 sleep in the sham group. N2: WMD 5.0 (95% CI: 4.3 to 5.7), p< 0.001, with an I2=40%. This suggests an increase in N2 sleep in the tDCS therapy group. N3/slow wave sleep: WMD -0.42 (95% CI: -0.77 to -0.07), p=0.02, I2=90%. This indicates an increase in N3/slow wave sleep in the sham group; 4) REM sleep phases: REM: WMD 1.48 (95% CI: 1.09 to 1.87), p<0.001, and an I2=62%. This suggests an increase in REM sleep in the tDCS therapy group. REM latency: WMD 3.09 (95% CI: 2.57 to 3.62), p<0.001, and an I2=91%. This indicates an increase in REM latency in the tDCS therapy group (Figures 5-7).

Forest plot of transcranial direct current stimulation objective sleep. SOL, sleep onset latency; TST, total sleep time.

Forest plot of transcranial direct current stimulation objective sleep. NREM, non-rapid eye movement sleep.

Forest plot of transcranial direct current stimulation objective sleep. REM, rapid eye movement; REML, rapid eye movement latency.
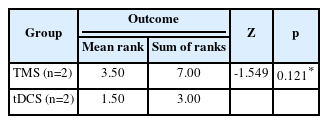
Based on the Mann-Whitney U test conducted based on subjective complaints in the two TMS studies and two tDCS studies, a result of Asymp. Sig (2-tailed) is 0.121. This result indicates that there is no significant difference between the two groups of intervention. The average rank in the TMS group is 3.5, while in the tDCS group is 1.5 (Table 2). In other words, the analysis did not find a statistically significant difference in the improvement of insomnia between the TMS and tDCS groups based on subjective complaints.
DISCUSSION
New therapy modalities like TMS and tDCS have been gradually developed over the years and implemented for treatments of insomnia [28]. Therefore, we conducted a meta-analysis to determine the effectiveness of TMS and tDCS in addressing insomnia and to investigate whether there are differences in the outcomes of insomnia between these two modalities. This systematic review and meta-analysis included a total of 245 individuals, with 118 participants in the TMS studies and 127 participants in the tDCS studies, divided into two groups: the active group and the sham group.
Psychological and behavioral interventions are recommended as effective treatments for comorbid insomnia. When initial psychological/behavioral treatment has been attempted but is ineffective, other psychological/behavioral therapies, combination Cognitive Behavioral Therapy for Insomnia therapy, or medication administration may be considered. In cases accompanied by comorbid depression or in instances of treatment failure, tranquilizing medications such as antidepressants or benzodiazepines may be considered [31]. A meta-analysis conducted by Zhou et al. [29] in 2020 found that despite the development of a significant number of antidepressant drugs, the response rates are still unsatisfactory. Regardless of the type of therapy, the primary goal of treatment is to improve the quality and quantity of sleep [32].
The results of the meta-analysis based on the PSQI assessment at week 4 showed that the use of TMS had a significant positive effect compared to the sham group, with a high level of heterogeneity. This heterogeneity can be caused by differences in the location, frequency, motor threshold of TMS stimulation, and the total session duration of TMS between the two studies. Additionally, the data used in the meta-analysis were not raw data from these two studies [23]. Consistent with the meta-analysis conducted by Zheng et al. [14], it was found that TMS is most effective when administered for an extended period of up to 4 weeks. This aligns with the findings of this study, where the research conducted by Pu et al. [28] demonstrated better results compared to the study conducted by Huang et al. [27] due to 4-week duration of TMS administration. Furthermore, the TMS stimulation location in the Pu et al. [28] study was in the left DLPFC region, which is considered a primary target area for insomnia comorbid depression [12,29].
The neural networks that regulate arousal and sleep consist of both bottom-up pathways and top-down pathways. Current pharmacological therapies for insomnia are relevant to some extent in addressing clinical conditions of arousal or sleep disturbances by modifying the neurotransmitter systems of the ascending reticular activating system (ARAS) (bottom-up pathways). However, their effectiveness is limited, and they come with varying side effects. This is complemented by the top-down corticothalamic pathways, which can be modified through NIBS techniques [6,18,19,33].
The results of the meta-analysis based on the subjective assessment of sleep quality in tDCS interventions using the components of PSQI and the sleep components of HAMD showed a slight improvement in insomnia compared to the sham group with high heterogeneity. The results of the meta-analysis based on objective sleep parameters using PSG showed a decrease in SOL, an increase in TST, N2, REM phase, and REM latency in the tDCS group, with moderate heterogeneity in N2 and REM phases. However, there was high heterogeneity in TST, N1, N3, and REM latency. These results collectively demonstrate the impact of tDCS therapy on various objective sleep parameters assessed by PSG in individuals with insomnia. While significant improvements were observed in some parameters, the high heterogeneity values suggest variability among the studies. This heterogeneity might be due to differences in the way antidepressant therapy was administered before and during the study and variations in the number of tDCS sessions between the two studies articles.
Based on the Mann-Whitney U test conducted based on subjective complaints in the two TMS studies and two tDCS studies, there was no significant difference between TMS and tDCS as effective approaches for insomnia comorbid depression with pvalue of 0.121. Both can be used as therapies for insomnia. However, the data obtained showed a higher average rating for TMS with mean rank 3.5 compared to tDCS with mean rank 1.5 in improving subjective sleep quality in insomnia comorbid depression. The non-significant difference might be caused by the limited number of articles, which was only two for each NIBS technique. Objective effectiveness comparison could not be performed because there was only one RCT study found for TMS, making it statistically inappropriate for comparison.
The strength of this study lies in the challenge of determining the types of insomnia suitable for conducting a meta-analysis. First, we included only RCT considered the gold standard, and gathered all RCT studies on the use of TMS and tDCS for insomnia in general to obtain as many articles as possible for meta-analysis. However, the types of insomnia in the RCT articles obtained were highly varied, with 26.7% of the articles about insomnia comorbid depression, 13.3% comorbid pain, 13.3% about insomnia in athletes, 13.3% primary insomnia, 6.7% drug-induced insomnia, 6.7% comorbid polio, 6.7% comorbid Sjogren’s syndrome, 6.7% comorbid stroke, and 6.7% comorbid Parkinson’s disease. This diversity in the types of research articles made it difficult to conduct a meta-analysis due to the high level of heterogeneity among the article samples, which could make significant bias. Second, we searched for similarities assessed based on the full text of each article to obtain the most homogeneous sample of RCT research articles and finding that 26.7% of the RCT research articles were about the use of TMS and/or tDCS in insomnia comorbid depression. As a result, the research team decided to conduct a meta-analysis based on the most abundant RCT articles with the most homogeneous research samples. For limitation of this study, first, the high heterogeneity resulting from differences in location parameters, stimulation, intervention duration, and variations in the duration of medication administration. Second, we only included published studies. Third, the sample size is small due to the very limited number of studies analyzed.
Although this study found no significant difference in the effectiveness of TMS and tDCS as therapies for insomnia; however, the data obtained indicate a higher average rating for TMS compared to tDCS in improving subjective sleep quality in insomnia with comorbid depression. The most prevalent type of insomnia observed in the use of TMS and tDCS as insomnia therapies was insomnia with comorbid depression. Both TMS and tDCS led to improvements in subjective insomnia parameters, while tDCS specifically resulted in improvements in objective insomnia parameters, including a reduction in SOL, and increase in TST, N2, REM, and REM latency among individuals with insomnia. Moving forward, the results of this meta-analysis can serve as valuable guidance for selecting these devices and can be used as a reference for future research endeavors. Additionally, the findings from this meta-analysis can provide individuals with an alternative to prevent drug dependency in managing their insomnia. It is also advisable to increase the number of primary RCTs focusing on TMS and tDCS in insomnia to facilitate more comprehensive meta-analytical studies with a larger pool of articles. Furthermore, future RCT research methods should aim to establish more uniform protocols for the application of TMS and tDCS in insomnia treatment.
Notes
Funding Statement
None
The authors have no potential conflicts of interest to disclose.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Devina Carolina Mastari, Junita Maja Pertiwi. Formal analysis: Devina Carolina Mastari, Windy Mariane Virenia Wariki. Investigation: Ansye Momole, Herlyani Khosama. Methodology: Devina Carolina Mastari, Windy Mariane Virenia Wariki. Software: Devina Carolina Mastari, Windy Mariane Virenia Wariki. Supervision: Finny Warouw, Junita Maja Pertiwi. Validation: Finny Warouw, Junita Maja Pertiwi. Writing—original draft: Devina Carolina Mastari, Windy Mariane Virenia Wariki. Writing—review & editing: all authors.
Acknowledgements
We would like to express our gratitude to Head of Department, Training Program Director and staff at Neurology Department Sam Ratulangi University for their invaluable guidance, support, and mentorship throughout the process. Last but not least, we would like to thank our families and friends for their support, encouragement, understanding, and constant source of motivation.