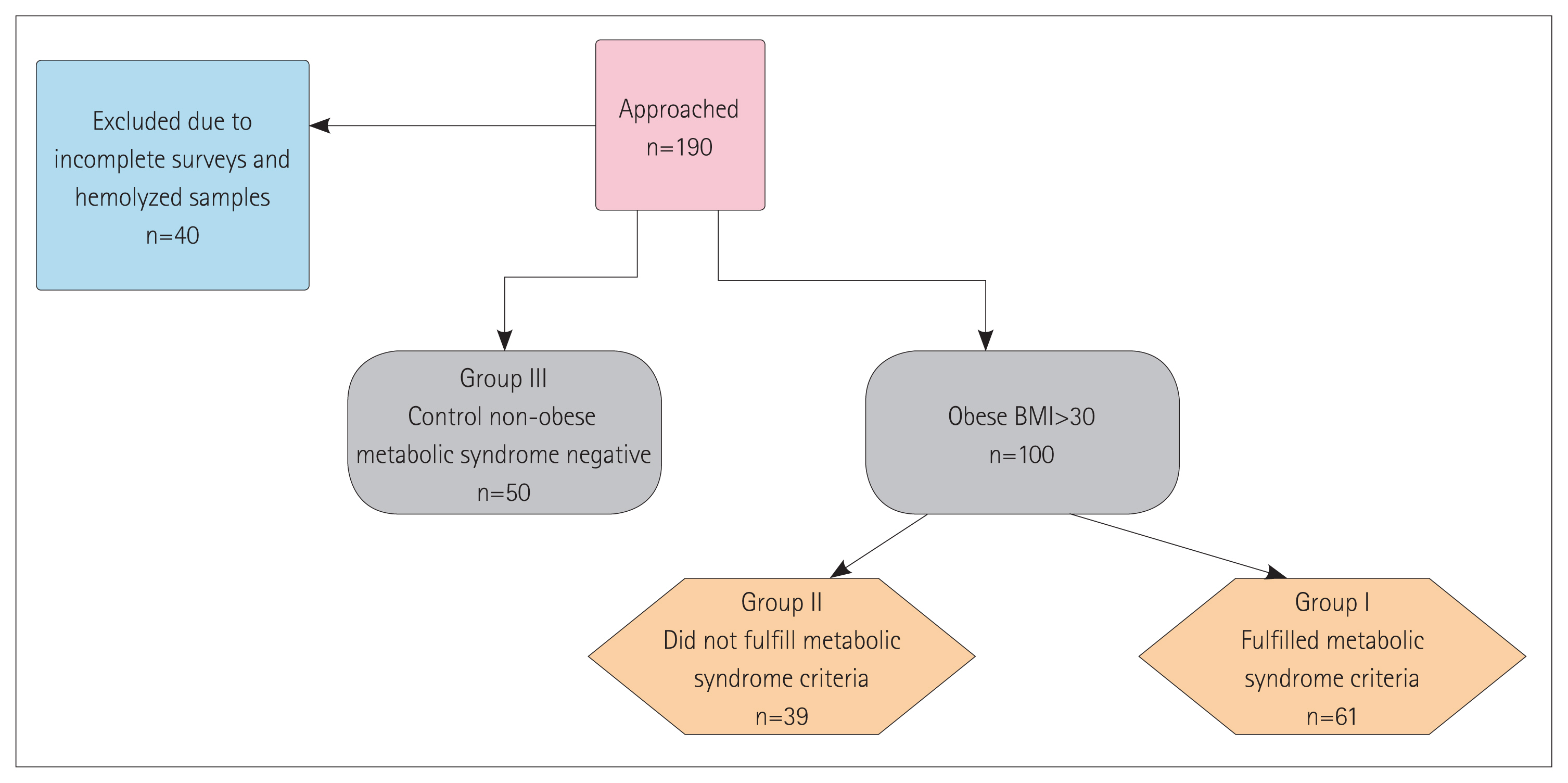
|
 |
- Search
| Chronobiol Med > Volume 2(4); 2020 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
Key Words
Metabolic syndrome; Obesity; Social jetlag; Chronotype, Night eating syndromeSupplementary Materials
Supplementary Figure 1
Acknowledgments
NOTES
Conflicts of Interest
Author Contributions
Conceptualization: Nevin Fayez Wanis Zaki, Amany Abdel Hamid Mousa, Naglaa Elsayed Abbas. Data curation: Yasmin Atwa Mohamed Ali. Formal analysis: Nevin Fayez Wanis Zaki. Investigation: Yasmin Atwa Mohamed Ali, Azza Abdelbaky. Methodology: Nevin Fayez Wanis Zaki, Amany Abdel Hamid Mousa, Naglaa Elsayed Abbas. Project administration: Amany Abdel Hamid Mousa, Ahmed Salem Bahammam. Resources: Nevin Fayez Wanis Zaki. Software: Nevin Fayez Wanis Zaki. Supervision: Amany Abdel Hamid Mousa, Naglaa Elsayed Abbas, Nevin Fayez Wanis Zaki, Ahmed Salem Bahammam. Validation: Nevin Fayez Wanis Zaki. Visualization: Nevin Fayez Wanis Zaki. Writing—original draft: Yasmin Atwa Mohamed Ali, Nevin Fayez Wanis Zaki. Writing—review & editing: Nevin Fayez Wanis Zaki, Ahmed Salem Bahammam.
Table 1
| Variable | Obese with MS (n=61) | Obese without MS (n=39) | Control (n=50) | KW/χ2 | p |
|---|---|---|---|---|---|
| Clinicodemographic variables | |||||
| Age (years) | 52 (44–55) | 41 (28–54) | 45 (31–51) | 19.927 | 0.03* |
| Sex, n (%) | 8.967 | 0.04* | |||
| Males | 30 (49.2) | 27 (69.2) | 33 (66) | ||
| Females | 31 (51.8) | 12 (30.8) | 17 (34) | ||
| BMI | 36.3 (32.4–40.3) | 34.7 (31.6–38.1) | 24.9 (22.8–27.3) | 98.79 | <0.0005* |
| WC (cm) | 107 (85–121) | 93 (78–109) | 75.5 (65.7–82.5) | 46.969 | <0.0005* |
| HC (cm) | 116 (89–134) | 100 (88–127) | 82 (70.75–97) | 34.308 | <0.0005* |
| WHR | 0.94 (0.89–0.96) | 0.92 (0.87–0.95) | 0.93 (0.82–0.97) | 2.435 | 0.296 |
| SBP (mm Hg) | 130 (120–140) | 120 (110–130) | 120 (110–120) | 24.355 | <0.0005* |
| DBP (mm Hg) | 80 (70–80) | 70 (70–80) | 70 (70–80) | 12.731 | 0.002* |
| MBP (mm Hg) | 96.7 (90–100) | 86.7 (83.3–93.3) | 88.3 (83.3–93.3) | 27.292 | <0.0005* |
| Laboratory variables | |||||
| FBG (mg/dL) | 168 (123–206) | 101 (100–123) | 89.5 (77–107) | 22.946 | <0.0005* |
| Creatinine (mg/dL) | 0.8 (0.7–1.1) | 0.9 (0.7–1) | 0.8 (0.7–1) | 1.816 | 0.403 |
| Albumin (gm) | 3.8 (3.4–4) | 4 (3.5–4) | 4 (3.8–4) | 16.205 | <0.0005* |
| AST (U/L) | 16 (14–22) | 15 (13–19) | 15 (12–18) | 5.608 | 0.061 |
| ALT (U/L) | 16 (14–21) | 17 (14–21) | 16 (15–18) | 0.493 | 0.781 |
| AST/ALT ratio | 0.94 (0.84–1.18) | 0.89 (0.8–1.04) | 0.89 (0.86–0.9) | 3.83 | 0.147 |
| TC (mg/dL) | 171 (143–207.5) | 146 (110–179) | 88.5 (77.7–99) | 85.391 | <0.0005* |
| LDL (mg/dL) | 93.6 (73–121.4) | 71.8 (41–112) | 15.6 (8.9–27.2) | 78.729 | <0.0005* |
| TG (mg/dL) | 166 (136.5–122) | 100 (90–124) | 90 (86.75–100) | 56.534 | <0.0005* |
| HDL (mg/dL) | 41 (35–46) | 51 (43–55) | 55 (48–65) | 52.889 | <0.0005* |
| Psychometric questionnaires | |||||
| Chronotype | 9.123 | 0.34 | |||
| Defenitely morning | 1 (1.6) | 2 (5.1) | 5 (10) | ||
| Moderately morning | 27 (44.3) | 13 (33.3) | 22 (44) | ||
| Neither | 26 (42.6) | 21 (53.8) | 18 (36) | ||
| Moderately evening | 5 (8.2) | 3 (7.7) | 5 (10) | ||
| Defenitely evening | 2 (3.3) | 0 (0) | 0 (0) | ||
| Total MEQ score | 17 (15–19) | 16 (13–18.5) | 18 (15–20) | 2.663 | 0.264 |
| Night Eating Questionnaire | 26.057 | <0.0005* | |||
| <25 | 30 (49.2) | 32 (82.1) | 45 (90) | ||
| 25–29 | 24 (39.3) | 5 (12.8) | 5 (10) | ||
| >30 | 7 (11.5) | 2 (5.1) | 0 (0) | ||
| Total score | 25 (21–27) | 22 (16–23) | 11 (14.5–22) | 37.528 | <0.0005* |
| Social jetlag | 0.312 | 0.856 | |||
| Present | 30 (49.2) | 17 (43.6) | 24 (48) | ||
| Absent | 31 (50.8) | 22 (56.4) | 26 (52) | ||
BMI: body mass index, WC: waist circumferernce, HC: hip circumferernce, WHR: waist/hip ratio, SBP: systolic blood pressure, DBP: diastolic blood pressure, MBP: mean arterial blood pressure, FBG: fasting blood glucose, AST: aspartate transaminase, ALT: alanine transaminase, TC: total cholesterol, LDL: low-density lipoprotein, TG: triglyceride, HDL: high-density lipoprotein. Data expression: median (IQR) [compared by Kruskal-Wallis H Test]. Data expression: frequency (percentage). P: chi-square test (Monte Carlo significance). Comparisons of column proportions (adjusted p-value using Bonferroni method).
Table 2
p-value: Spearman’s correlation. MS: metabolic syndrome, BMI: body mass index, WC: waist circumference, HC: hip circumference, WHR: waist to hip ratio, MBP: mean arterial blood pressure, FBG: fasting blood glucose, TC: total cholesterol, LDL: low-density lipoprotein, TG: triglyceride, HDL: high-density lipoprotein, MEQ: Morningness-Eveningness Questionnaire, NEQ: Night Eating Questionnaire
Table 3
Table 4
Adjusted for age and sex. Cutoff values were depicted by ROC curve analyses (Supplementary Figure 1). NEQ: Night Eating Questionnaire, B: binary logistic regression coefficient, SE: standard error, OR: odds ratio, CI: confidence interval
REFERENCES
- TOOLS